The Evolution of Refrigerants Towards Carbon Neutrality
These days we often take for granted that our food will be transported and stored to maintain peak ripeness and freshness. We’re comfortable enough to drive our car on a sweltering summer day, knowing we’ll stay cool and dry. Even in the hottest places on earth, we know we can work and live comfortably in temperature-controlled buildings.
While the pandemic has put a strain on our global infrastructure, it also brought the business and science of refrigeration to greater public consciousness. We’re all aware that certain of the approved vaccines require storage and transport at temperatures reaching -80oC.

This story is written by Maersk Container Industry and draws information from various historical and scientific sources.
Focus on refrigerants
The history and science behind keeping food fresh and air cool goes back thousands of years. Before mechanical refrigeration systems were introduced, people cooled their food with ice. Transported from mountains or nearby frozen bodies of water , ice was stored underground in snow cellars insulated with wood and straw. Blocks of ice delivered to homes provided the means of keeping food chilled in non-mechanical refrigerators or ice boxes.
In the 1740’s, the first form of artificial refrigeration was invented by William Cullen, a Scottish scientist. Cullen showed how the rapid heating of liquid to a gas can result in cooling. This is the principle behind refrigeration that remains today. Cullen never turned his theory into practice.
In 1802 Thomas Moore, an American businessman, created an icebox to cool dairy products for transport. He called it a “refrigiratory” until he patented “refrigerator” in 1803.
In 1834 American inventor Jacob Perkins, living in London at the time, built the world’s first working vapor-compression refrigeration system, using ether in a closed cycle. His prototype system worked and was the first step to modern refrigerators, but it didn’t succeed commercially.
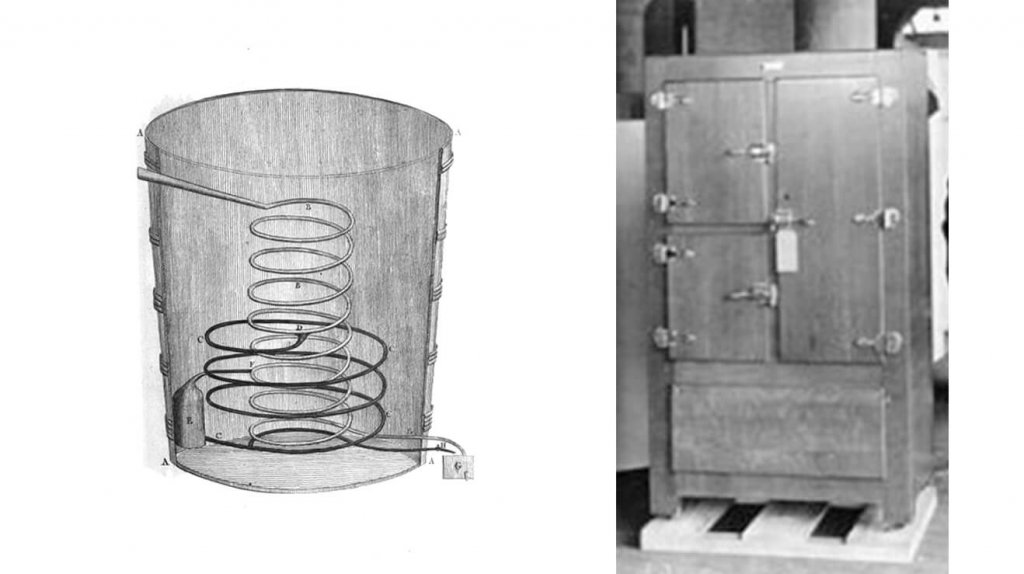
In 1876 German engineering professor Carl von Linde patented the process of liquefying gas that has become part of basic refrigeration technology. His findings led to his invention of the first reliable and efficient compressed-ammonia refrigerator. Refrigeration rapidly displaced ice in food handling and was introduced into many industrial processes.
Refrigerants are the working medium used in refrigerating and cooling systems. Modern refrigeration systems essentially all work the same way – by passing a refrigerant between the main components of the compressor, condenser, expansion device and the evaporator to move unwanted heat from one location to another. A refrigerant is a fluid that can easily boil from a liquid into a vapor and be condensed from a vapor back into a liquid. The process of refrigeration requires that this occurs again and again, continuously without fail.
Between the late 1800’s and 1929, mechanical refrigeration used natural refrigerants such as methyl chloride (R40), ammonia and sulfur dioxide. With little concern for safety, the focus was on what worked and what was readily available. Nearly all the first-generation refrigerants were flammable, toxic or both and some were also highly reactive.
There were numerous fatal accidents that occurred in the 1920s when methyl chloride leaked out of refrigerators. Methyl chloride proved to be the root cause of a rash of reefer container explosions and a number of deaths seen in the 2000s, resulting from the use of counterfeit R134a.
While refrigeration plays a vital role in domestic and industrial application, the refrigerants used to achieve the intended results have also contributed to the world’s major environmental issues like ozone depletion and global warming. The modern history behind the development and eventual evolution of greener refrigerants mostly lies in the work of two 20th century chemists; one who forged a reliable method of mechanical refrigeration and the other who made the world aware of the environmental dangers associated with refrigerants.

Thomas Midgley, Jr., the first of our 20th century chemists, was an American engineer and chemist who while working for General Motors in 1921 discovered a solution to engine knock in high-compression gasoline engines. Midgley’s team discovered that the addition of tetraethyl lead to gasoline eliminated engine knock and improved gas combustion. Undaunted by the known toxicity of lead, Midgley never wavered in his conviction that tetraethyl lead could be produced safely and that the small amount of lead particulates expelled in engine exhaust would not pose a threat to public health.
In 1930, Midgley was challenged to find an odor-free, non-toxic, and nonflammable refrigerant gas that could be used in commercial refrigerators and air conditioners. Studying the property of elements on the periodic table, he disregarded compounds that are unstable, toxic, yielding insufficient volatility and the inert gases based on their low boiling point. His research focused on the flammability and toxicity of compounds containing elements like carbon, nitrogen, oxygen, sulfur, hydrogen, fluorine, chlorine and bromine. His first publication was on fluorochloro refrigerants and it showed how the variation of chlorination and fluorination of hydrocarbons influenced boiling point, flammability, and toxicity of the refrigerants. In short time, Midgley settled on dichlorodifluoromethane, commercially produced as Freon-12. Thus, the birth of CFC refrigerants as the 2nd generation of refrigerants.
Midgley was a brilliant man driven to find solutions to the unique problems and circumstances of his time. While much was gained by his discoveries, the toll taken on the environment is perhaps immeasurable. History likely remembers him more cruelly, if at all. In an odd twist of fate, Midgley died at the young age of 55 of his own ingenuity. Having contracted polio in 1940 and losing the use of his legs, Midgley invented a hoist mechanism that helped him in and out of bed. In 1944, he died of strangulation in that very hoist mechanism.
Midgley’s discovery proved CFC as a good refrigerant because it can be compressed easily to liquid and carries away lots of heat when it evaporates, while remaining very stable and non-explosive. As a highly versatile refrigerant, it found use for a wide range of refrigeration and air conditioning applications for both commercial and home use. What wasn’t known at the time is that CFCs deplete the ozone layer.

This brings us to Dr. James Lovelock, the second of our 20th century chemists. Dr. Lovelock is an English chemist, medical doctor, scientific instrument developer and noted author best known for the creation and promulgation of the Gaia hypothesis – an idea rooted in the notion that all life on Earth is part of an entity that regulates Earth’s surficial and atmospheric processes.

Source: The Guardian
In 1957, Lovelock was working at the National Institute for Medical Research (NIMR), and invented an early version of the electron-capture detector (ECD) – a device used in gas chromatography that draws upon the ionization properties of argon to detect trace atoms and molecules in a gas sample. The ECD has been used to determine the concentrations of halogen compounds in food and in the atmosphere, including compounds associated with residues of the pesticide DDT and with polychlorinated biphenyls (PCBs) and CFCs.
In 1970, Lovelock was using the ECD to detect CFCs in the urban haze of pollution that had descended on his holiday home in Ireland. He found CFCs all around him. Lovelock even took his instrument on a voyage from England to Antarctica and was surprised to find CFCs wherever he travelled. It seemed that CFCs weren’t being broken down in the Earth’s lower atmosphere. In fact, almost all of the CFCs manufactured since their invention in 1930 were still in the atmosphere.
A few years later, atmospheric chemists Sherwood Rowland and Mario Molina made the hypothesis that these stable CFCs might eventually float into the Earth’s upper atmosphere, known as the stratosphere, where solar radiation would break down the molecules and release highly reactive chlorine atoms. These chlorine atoms could, in turn, catalyze the destruction of ozone at a devastating rate. A single chlorine atom could destroy 100,000 ozone molecules.
From there, the evidence mounted. Chlorine monoxide, a product from which the only known source is the destruction of ozone by chlorine, was detected in the stratosphere in 1976. A year later, the governments of the U.S., Canada, Norway and Sweden moved to phase out CFCs from aerosols. It would be another ten years before the Montreal Protocol was signed (the first United Nations treaty to be signed by all 197 member nations) and CFCs would begin to be phased out from all products.
As of this writing, Dr. Lovelock is still with us. In July of 2020 he celebrated his 101st birthday and remains a staunch advocate of environmental initiatives.

We’ve known since the 1970s, that the release of certain gasses into the atmosphere resulted in erosion of the Earth’s ozone layer and contributed to global warming. Unfortunately, it took 50 years for the science to catch up with the damage done by the gas compounds in the very refrigerants used to cool our homes and keep our food fresh since the 1930s.
Greenhouse gases warm the Earth by absorbing energy and slowing the rate at which the energy escapes into space. A greenhouse gas is defined as any of the various gaseous compounds (such as carbon dioxide or methane) that absorb infrared radiation, trap heat in the atmosphere and contribute to the greenhouse effect.
Global initiatives such as the Montreal Protocol, the Kyoto Protocol and later the Paris Climate Agreement have mandated a phased down reduction and eventual elimination of production and use of Ozone Depleting Chemicals (ODCs). Refrigerants came under scrutiny as significant contributors to the greenhouse effect.
These gases and their impact on global warming are measured and compared by their Global Warming Potential (GWP). GWP is a measure of how much energy the emissions of 1 ton of a gas will absorb over a given period to time, relative to the emission of 1 ton of carbon dioxide (CO2). As reference, dichlorodifluoromethane (R-12 or CFC-12, the most used Freon brand refrigerant prior to its ban in many countries in 1996 and total ban in 2010) has a GWP of 10,900 – meaning 10,900 times the environmental impact of CO2.
A 100-year period has been the standard used to measure absorption of gas emissions. However, the Intergovernmental Panel on Climate Change (IPPC) also publishes a 20-year timeline for GWPs and there is ongoing discussion if this shorter timeline provides a more prescient snapshot.
The Montreal Protocol forced respective industries to develop alternative refrigerants with an ever-lower GWP. Tetrafluoroethane (R-134a or HCFC-134a) first appeared in the early 1990s and has been one of the main replacements for the formerly widespread R-12. The GWP for R-134a is 1430 compared to R-12’s GWP of over 10,000.
In 2006, European Union directive 2006/40/EC addressed auto a/c emission levels with an effective date of 2011. The directive required that all new cars for sale in Europe use a refrigerant in its a/c system with a GWP below 150. This led to Dupont’s development of Tetrafluoropropene, HFO-1234yf (R-1234yf). R-1234yf is a hydrofluoroolefin and the first of a new class of refrigerants. R-1234yf surpassed the EU mandate, achieving a GWP of less than 1 in the IPPC’s 5th assessment report. The previous assessment rated R-1234yf’s GWP at 4.
For MCI, our Star Cool machines have been engineered to work alternatively with either R134a or R513A since 2017. R513A is a blend of R134a and R1234yf and has a GWP of 631 (a 56% reduction versus R134a).
There’s a huge amount of history, science and engineering that’s gone into allowing the precise carriage of fresh avocados half-way around the world or allows you to jump into your car on a hot summer’s day knowing you’ll be comfortable. The science and technology continue to evolve to meet the challenges of improving the environment.

Learn more
By adjusting injection control, air flow and software for R513A compatibility, Star Cool offers an economically sound upgrade that can achieve a 56% reduction of GWP compared to the previously used R134a (GWP=1430). Among all A1 classified F-gases, R513A is the best choice to reduce the carbon footprint of reefer containers today.


